Features & Benefits
- Proven MLCC technology appropriate for medical applications
- Internal reliability qualification and lot acceptance testing (LAT) to support medical application requirements
- Defined designs, materials, processes, and manufacturing facilities with change control that meets FDA requirements
Typical Applications
- Implantable, Non-Life Sustaining Medical Devices (e.g. implanted temporary cardiac monitor, insulin pumps)
- External, Life Sustaining Medical Devices (e.g. heart pump external controller)
- External Devices (e.g. patient monitoring, diagnostic equipment)
The Wexton Line MM series is a multi-layer ceramic capacitor designed for use in medical applications other than implantable/life support. These components have the design & change control expected for medical devices and also offer enhanced LAT including reliability testing and 100% inspection.
Datasheet / Catalog – Click to Download
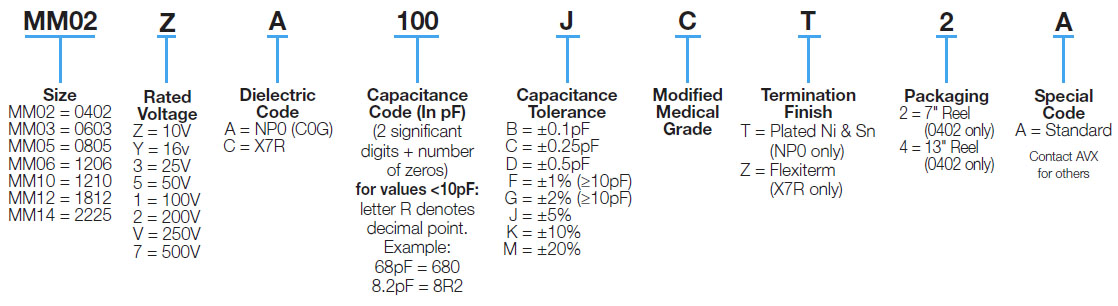
Part Number Information